Case Report: Drug-Induced Sweet Syndrome Following Inhalation Therapy Change for COPD
This case highlights the importance of clinical vigilance in primary care, especially when new symptoms arise after changes in medication.
The patient, with a history of hypertension and chronic obstructive pulmonary disease (COPD), had no known allergies. She was a smoker, averaging ten cigarettes daily, and had been taking enalapril for six years and inhaled formoterol for two. Due to worsening respiratory symptoms, her pulmonologist switched her to a combination of indacaterol and glycopyrronium inhaled capsules.
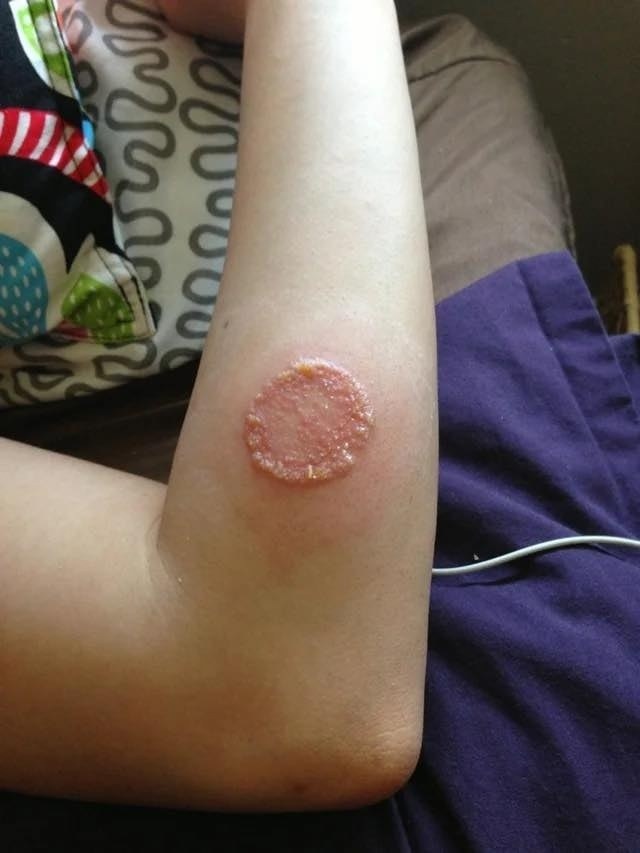
Just two days after starting the new medication, the patient developed painful, red (erythematous) patches on her cheeks and neck, along with a mild fever. She reported no recent illness, no changes in skincare or diet, and had used sunscreen during recent sun exposure. Given the sudden and severe onset of the skin lesions, her primary care physician referred her urgently to Dermatology.
The dermatologists recommended discontinuation of the new inhaler and prescribed oral corticosteroids. A skin biopsy was performed, alongside a full panel of blood tests, including a complete blood count, autoimmune markers, lupus anticoagulant, and serology. Within 48 hours, the skin lesions began to improve and her pain significantly reduced. Blood work showed leukocytosis with neutrophilia, but all autoimmune and infectious tests were negative.
Twenty days later, the biopsy confirmed a diagnosis of Sweet syndrome, a rare inflammatory skin condition marked by the sudden appearance of painful, red plaques or papules, usually with fever and elevated white blood cell count.
Understanding Sweet Syndrome
Sweet syndrome belongs to a group of disorders called neutrophilic dermatoses, characterized by a dense infiltration of neutrophils into the skin. Lesions typically present as tender, red or purple papules and plaques—often located on the face, neck, arms, or upper torso. Systemic symptoms like fever and fatigue are frequently present.
The exact cause of Sweet syndrome is unknown, but it is believed to result from an abnormal immune response, with cytokine release attracting neutrophils to the skin. Triggers may include infections, autoimmune conditions, malignancies (especially blood cancers), and certain medications.
In this case, the timing strongly pointed to a drug-induced Sweet syndrome, likely caused by the recently introduced indacaterol/glycopyrronium inhaler. While several medications have previously been linked to Sweet syndrome—such as contraceptives, antiepileptics, antibiotics, antihypertensives, vaccines, and growth factors—this represents the first reported case involving this specific inhaled drug combination.
Diagnosis and Treatment
Diagnosis relies on the clinical presentation, lab findings indicating systemic inflammation (fever, leukocytosis, neutrophilia), and confirmation via biopsy, which shows dense neutrophilic infiltrates in the dermis. The differential diagnosis includes urticaria, contact dermatitis, drug eruptions, and lupus erythematosus.
The first-line treatment is systemic corticosteroids, which usually produce a rapid response—often within hours. Fever and pain subside quickly, and skin lesions typically resolve within a week. For recurrent or resistant cases, alternative treatments such as colchicine, dapsone, or potassium iodide may be used.
In this case, stopping the suspected drug and starting corticosteroids led to a fast and full recovery.
Clinical Implications
Although rare, Sweet syndrome should be considered in patients presenting with sudden, painful skin eruptions alongside fever or leukocytosis—especially after initiating a new medication. Prompt recognition and withdrawal of the suspected drug are crucial to avoid complications or recurrence.
Physicians should also evaluate for underlying conditions, as Sweet syndrome may be an early sign of autoimmune disorders, infections, or malignancies.
This case serves as a reminder that even inhaled medications, typically considered low-risk, can provoke systemic immune responses. It underscores the importance of monitoring patients carefully after treatment changes and referring them promptly when unexpected adverse effects occur.
Ethical Considerations
The authors confirm compliance with all ethical standards. No experiments were conducted on humans or animals, and the patient provided informed consent for publication of the case.
Conclusion
Sweet syndrome is an uncommon but potentially serious condition that is often misdiagnosed in its early stages. Primary care providers play a key role in identifying unusual skin reactions, linking them to possible drug exposure, and ensuring timely referrals.
This case—the first to associate Sweet syndrome with inhaled indacaterol and glycopyrronium—emphasizes the importance of remaining alert to unexpected drug reactions, regardless of how rare they may be.